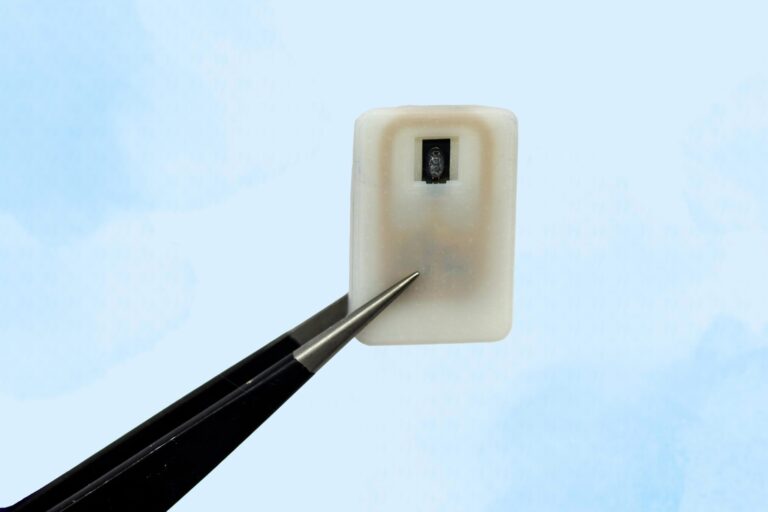
The new implant contains a reservoir of glucagon that sits beneath the skin and can be activated in an emergency, with no need for injections.
For individuals with Type 1 diabetes, the risk of hypoglycemia, dangerously low blood sugar, is a constant concern. When glucose levels drop too far, the condition becomes life-threatening and typically requires an injection of the hormone glucagon as the standard treatment.
To address situations where patients may be unaware that their blood sugar is falling to critical levels, Emergency response
Many people with type 1 diabetes rely on daily Each unit can store one or four doses of glucagon and contains an antenna that responds to a specific radiofrequency signal. When triggered, the antenna activates a small electrical current that heats the shape-memory alloy. Once the material reaches the activation temperature, it bends into a U shape and releases the powdered drug from the reservoir. Because the device can receive wireless signals, it could also be designed so that drug release is triggered by a glucose monitor when the wearer’s blood sugar drops below a certain level. “One of the key features of this type of digital drug delivery system is that you can have it talk to sensors,” Krishnan says. “In this case, the continuous glucose-monitoring technology that a lot of patients use is something that would be easy for these types of devices to interface with.” After implanting the device in diabetic mice, the researchers used it to trigger glucagon release as the animals’ blood sugar levels were dropping. Within less than 10 minutes of activating the drug release, blood sugar levels began to level off, allowing them to remain within the normal range and avert hypoglycemia. The researchers also tested the device with a powdered version of epinephrine. They found that within 10 minutes of drug release, epinephrine levels in the bloodstream became elevated and heart rate increased. In this study, the researchers kept the devices implanted for up to four weeks, but they now plan to see if they can extend that time up to at least a year. “The idea is you would have enough doses that can provide this therapeutic rescue event over a significant period of time. We don’t know exactly what that is — maybe a year, maybe a few years, and we’re currently working on establishing what the optimal lifetime is. But then after that, it would need to be replaced,” Krishnan says. Typically, when a medical device is implanted in the body, scar tissue develops around the device, which can interfere with its function. However, in this study, the researchers showed that even after fibrotic tissue formed around the implant, they were able to successfully trigger the drug release. The researchers are now planning for additional animal studies and hope to begin testing the device in clinical trials within the next three years. “It’s really exciting to see our team accomplish this, which I hope will someday help diabetic patients and could more broadly provide a new paradigm for delivering any emergency medicine,” says Robert Langer, the David H. Koch Institute Professor at MIT and an author of the paper. Reference: “Emergency delivery of particulate drugs by active ejection using in vivo wireless devices” by Siddharth R. Krishnan, Laura O’Keeffe, Arnab Rudra, Derin Gumustop, Nima Khatib, Claudia Liu, Jiawei Yang, Athena Wang, Matthew A. Bochenek, Yen-Chun Lu, Suman Bose, Kaelan Reed, Robert Langer and Daniel G. Anderson, 9 July 2025, Nature Biomedical Engineering. The research was funded by the Leona M. and Harry B. Helmsley Charitable Trust, the Join the SciTechDaily newsletter.
Reversing hypoglycemia
DOI: 10.1038/s41551-025-01436-2